Difference between revisions of "Macrophage galactose lectin (MGL)"
| (28 intermediate revisions by 5 users not shown) | |||
| Line 3: | Line 3: | ||
== CFG Participating Investigators contributing to the understanding of this paradigm == | == CFG Participating Investigators contributing to the understanding of this paradigm == | ||
In addition to creating the knockout for the two mouse forms of MGL, PIs have been involved in extensive studies of binding specificity and mechanism of ligand binding as well as the role of the receptor in macrophage signaling. | In addition to creating the knockout for the two mouse forms of MGL, PIs have been involved in extensive studies of binding specificity and mechanism of ligand binding as well as the role of the receptor in macrophage signaling. | ||
| − | * PIs working on MGL include: Nicolai Bovin, Kurt Drickamer, Toshisuke Kawasaki, Cheng Liu, Yvette van Kooyk, Hui Wu | + | * PIs working on MGL include: Nicolai Bovin, Kurt Drickamer, Toshisuke Kawasaki, Cheng Liu, Yvette van Kooyk, Hui Wu, Joy Burchell,Joyce Taylor-Papadimitriou |
* Non-PIs with who have used CFG resources to study MGL include: Siamon Gordon, Alan Saltiel | * Non-PIs with who have used CFG resources to study MGL include: Siamon Gordon, Alan Saltiel | ||
* PIs working on MGL-related glycan-binding proteins (GBPs), particularly Mincle, include: Anthony dApice, Joshua Fierer, Rikard Holmdahl, Christopher O'Callaghan, Judy Teale, Christine Wells | * PIs working on MGL-related glycan-binding proteins (GBPs), particularly Mincle, include: Anthony dApice, Joshua Fierer, Rikard Holmdahl, Christopher O'Callaghan, Judy Teale, Christine Wells | ||
| Line 9: | Line 9: | ||
== Progress toward understanding this GBP paradigm == | == Progress toward understanding this GBP paradigm == | ||
| + | This section documents what is currently known about MGL, its carbohydrate ligand(s), and how they interact to mediate cell communication. Further information about MGL can be found in its [http://www.functionalglycomics.org/glycomics/molecule/jsp/viewGbpMolecule.jsp?gbpId=cbp_hum_Ctlect_217&sideMenu=no GBP Molecule Page] in the CFG database. | ||
| + | === Carbohydrate ligands === | ||
| + | *mMGL1 binds Lewis X and Lewis A structures, whereas mMGL2 recognizes N-acetylgalactosamine (GalNAc) and galactose, including the O-linked Tn-antigen and TF-antigen <ref name=Tsuiji 2002"/><ref name="Singh 2009"/><Ref name="Napoletano2007"/> | ||
| + | *hMGL binds terminal α- and β-linked GalNAc residues on glycoproteins, glycolipids and bacterial LPS, including Tn antigen and GalNAcβ1-4GlcNAc-R (LDN) antigens <ref name="Suzuki 1996"/><ref name="Van Vliet 2005">van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, Geijtenbeek TB, Blixt O, Alvarez R, van Die I and van Kooyk Y. 2005. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 17:661-669</ref><ref>van Sorge NM, Bleumink NM, van Vliet SJ, Saeland E, van der Pol WL, van Kooyk Y and van Putten JP. 2009. N-glycosylated proteins and distinct lipooligosaccharide glycoforms of Campylobacter jejuni target the human C-type lectin receptor MGL. Cell Microbiol. 11:1768-1781</ref><ref>Saeland E, van Vliet SJ, Backstrom M, van den Berg VC, Geijtenbeek TB, Meijer GA and van Kooyk Y. 2007. The C-type lectin mgl expressed by dendritic cells detects glycan changes on Muc1 in colon carcinoma. Cancer Immunol Immunother. 56:1225-1236</ref><ref name="Napoletano2007">Napoletano C, Rughetti A, Tarp M.P.A, Coleman J Bennett,E.P, Picco G, Sale P, Denda-Hagai K, Irimura T, Mandel U, Clausen H, Frati L, Taylor-Papadimitriou J, Burchell J, Nuti M. Tumour associated Tn-MUC1 glycoform is internalised througfh the macrophage galactose C-type lectin and delivered to the HLA class I and Class II compartments in dendritic cells. Cancer Research, 2007, 67(17): 8358-8367</ref>. | ||
| + | *hMGL can also bind to STn when presented on a peptide or polyacrylamide backbone. | ||
| − | |||
| − | |||
| − | |||
| − | |||
=== Cellular expression of GBP and ligands === | === Cellular expression of GBP and ligands === | ||
| − | MGL is expressed on | + | MGL is expressed on dendritic cells and macrophages. <ref name="Higashi 2002"/><ref name=" van Vliet SJ1200 ">van Vliet SJ, Gringhuis SI, Geijtenbeek TB and van Kooyk Y. 2006. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 7:1200-1208</ref> |
<br> | <br> | ||
| − | === Biosynthesis of | + | The Tn ligand is expressed by many cancer cells especially breast cancers where it is expressed on more than 90% of breast carcinomas <ref> Sørensen AL, “et al”. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. “Glycobiology” 16, 96-107 (2006) </ref>. although STn is also expressed by carcinomas, especially colorectal and in 25-30% of breast cancers. |
| + | |||
| + | === Biosynthesis of ligands === | ||
| + | The Tn ligand can be expressed in cervical cancer due to mutations in Cosmc <ref> Ju T, “et al”. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc “Cancer Research” 68, 1636-1646 (2008) </ref>. a molecular chaperone that is essential for the activity of the T synthase, the glycosyltransferase that catalyses the addition Gal to GalNAcαSer/Thr, forming the T antigen (Galβ1,3GalNAcαSer/Thr). | ||
| + | Although the vast majority of breast cancers express Tn there is no evidence of mutated Cosmc in these cancers therefore another mechanism for the expression of the Tn ligand must be active. Moreover the expression of STn in breast cancer is perfectly correlated with the turning on of the transcription of ST6GalNAc-I <ref> Sewell R, “et al”. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. “J Biol Chem” 281, 3586-3594 (2006) </ref>. | ||
<br> | <br> | ||
| + | |||
=== Structure === | === Structure === | ||
| + | [[image:MGL.jpg]]<br> | ||
| + | MGL is an oligomeric type II transmembrane protein. The CRD of the major subunit of the hepatic asialoglycoprotein receptor has been determined<ref name="Meier2000">Meier, M, Bider, MD, Malashkevich, VN, Spiess, M and Burkhard, P. 2000. Crystal structure of the carbohydrate recognition domain of the H1 subunit of the asialoglycoprotein receptor. J Mol Biol 300:857–865</ref> and the structure of a galatose-binding mutant of mannose-binding protein provides experimental evidence for how galactose- and GalNAc-terminated ligands can bind to the receptor.<ref name="Kolatkar2000">Kolatkar, AR, Leung, AK, Isecke, R, Brossmer, R, Drickamer, K and Weis, WI. 1998. Mechanism of N-acetylgalactosamine binding to a C-type animal lectin carbohydrate-recognition domain. J Biol Chem 273:19502-19508</ref> | ||
| + | <br> | ||
| − | |||
=== Biological roles of GBP-ligand interaction === | === Biological roles of GBP-ligand interaction === | ||
*MGL is a highly efficient internalization receptor <ref name="Higashi 2002"/><ref>Valladeau J, Duvert-Frances V, Pin JJ, Kleijmeer MJ, Ait-Yahia S, Ravel O, Vincent C, Vega F, Jr., Helms A, Gorman D, Zurawski SM, Zurawski G, Ford J and Saeland S. 2001. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J Immunol. 167:5767-5774</ref> | *MGL is a highly efficient internalization receptor <ref name="Higashi 2002"/><ref>Valladeau J, Duvert-Frances V, Pin JJ, Kleijmeer MJ, Ait-Yahia S, Ravel O, Vincent C, Vega F, Jr., Helms A, Gorman D, Zurawski SM, Zurawski G, Ford J and Saeland S. 2001. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J Immunol. 167:5767-5774</ref> | ||
| − | *hMGL regulates T-cell receptor mediated signaling and T-cell dependent cytokine responses <ref name=" van Vliet SJ1200 "/ | + | *hMGL regulates T-cell receptor mediated signaling and T-cell dependent cytokine responses <ref name=" van Vliet SJ1200 "/> |
*mMGL1 promotes adipose tissue inflammation and insulin resistance <ref>Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. 2009. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med 206: 3143-56</ref> | *mMGL1 promotes adipose tissue inflammation and insulin resistance <ref>Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. 2009. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med 206: 3143-56</ref> | ||
| − | *mMGL2 promotes enhances both MHC class II and class I presentation antigen in | + | *mMGL2 promotes enhances both MHC class II and class I presentation antigen in dendritic cells (DCs) <ref>Singh SK, Streng-Ouwehand I, Litjens M, Kalay H, Saeland E, Van Kooyk Y. 2010. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int. J. Cancer, in press</ref> |
<br> | <br> | ||
| + | |||
== CFG resources used in investigations == | == CFG resources used in investigations == | ||
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=MGL&maxresults=20 CFG database search results for MGL]. | The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=MGL&maxresults=20 CFG database search results for MGL]. | ||
| Line 36: | Line 45: | ||
<br> | <br> | ||
=== Glycogene microarray === | === Glycogene microarray === | ||
| + | Probes for the single human MGL and both mouse MGLs have been included in all versions of the CFG glycogene chip. | ||
| + | <br> | ||
| − | |||
=== Knockout mouse lines === | === Knockout mouse lines === | ||
| − | + | Mice lacking MGL-1<ref name=Onami2002>Onami, TM, Lin, M-Y, Page, DM, Shirley A. Reynolds, SA, Katayama, CD, Marth, JD, Irimura, T, Varki, A, Varki, N and Hedrick SM (2002) Generation of mice deficient for dacrophage galactose- and N-acetylgalactosamine-specific lectin: limited role in lymphoid and erythroid homeostasis and evidence for multiple lectins. Mol. Cell. Biol. 22, 5173-5181</ref> were distributed by the CFG and the [https://www.functionalglycomics.org/glycomics/publicdata/phenotyping.jsp phenotype] was analyzed. Mice lacking MGL-2 have also been described.<ref name="Denda2010">Denda-Nagai, K, Aida, S, Saba, K, Suzuki, K, Moriyama, S, Oo-puthinan, S, Tsuiji, M, Morikawa, A, Kumamoto, Y, Sugiura, D, Kudo, A, Akimoto, Y, Kawakami, H, Bovin NV, and Irimura, T (2010) Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J. Biol. Chem. 285, 19193–19204</ref> ES cells for an MGL-1/2 double knockout, generated in Core F, are available from [http://www.functionalglycomics.org/static/consortium/resources/resourcecoref.shtml#table1 MMRRC]. | |
=== Glycan array === | === Glycan array === | ||
| − | The glycan-binding specificity of | + | The glycan-binding specificity of [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_GLYCAN_v2_13_11262003 human] and [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_2010 mouse] versions of MGL have been analyzed by glycan array screening <ref name="Van Vliet 2005"/><ref name="Singh 2009"/>. See all glycan array results for MGL [http://www.functionalglycomics.org/glycomics/search/jsp/result.jsp?query=mgl&cat=coreh here]. See glycan array results for these related GBPs: [http://www.functionalglycomics.org/glycomics/search/jsp/result.jsp?query=mannose%20AND%20receptor%20NOT%20asialoglycoprotein&cat=coreh mannose receptor,] [http://www.functionalglycomics.org/glycomics/search/jsp/result.jsp?query=mincle&cat=coreh mincle,] [http://www.functionalglycomics.org/glycomics/search/jsp/result.jsp?query=MCL&cat=coreh macrophage C-type lectin (MCL),] and [http://www.functionalglycomics.org/glycomics/search/jsp/result.jsp?query=dectin-1&cat=coreh dectin-1.] |
== Related GBPs == | == Related GBPs == | ||
| − | Other C-type lectins on macrophages include the mannose receptor, mincle <ref>Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, Schroder K, Ma B, Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180: 7404-7413</ref>, macrophage C- type lectin (MCL), and dectin-1. | + | Other C-type lectins on macrophages include the mannose receptor [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=DC-SIGNR&maxresults=20 (CFG data)], mincle <ref>Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, Schroder K, Ma B, Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180: 7404-7413</ref> [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=mincle&maxresults=20 (CFG data)], macrophage C- type lectin (MCL) [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=MCL&maxresults=20 (CFG data)], and dectin-1 [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=dectin-1&maxresults=20 (CFG data)]. |
== References == | == References == | ||
| Line 52: | Line 62: | ||
== Acknowledgements == | == Acknowledgements == | ||
| − | The CFG is grateful to the following PIs for their contributions to this wiki page: Kurt Drickamer, Yvette van Kooyk | + | The CFG is grateful to the following PIs for their contributions to this wiki page: Kurt Drickamer, Joyce Taylor-Papadimitriou, Yvette van Kooyk, Irma van Die |
Latest revision as of 09:02, 16 April 2011
Macrophage galactose binding lectin (MGL) is the best studied of the multiple C-type lectins on macrophages [1][2]. It is also representative of the subclass of C-type lectins that bind galactose-related sugars. MGL consists of one CRD domain and contains cytoplasmic internalization motifs for endocytosis. No signaling properties have been described yet for MGL. Human MGL (CD301) and rat MGL are encoded by a single gene, whereas mice contain two MGL copies, mMGL-1 and mMGL-2 that differ in carbohydrate specificity [3][4][5].
CFG Participating Investigators contributing to the understanding of this paradigm
In addition to creating the knockout for the two mouse forms of MGL, PIs have been involved in extensive studies of binding specificity and mechanism of ligand binding as well as the role of the receptor in macrophage signaling.
- PIs working on MGL include: Nicolai Bovin, Kurt Drickamer, Toshisuke Kawasaki, Cheng Liu, Yvette van Kooyk, Hui Wu, Joy Burchell,Joyce Taylor-Papadimitriou
- Non-PIs with who have used CFG resources to study MGL include: Siamon Gordon, Alan Saltiel
- PIs working on MGL-related glycan-binding proteins (GBPs), particularly Mincle, include: Anthony dApice, Joshua Fierer, Rikard Holmdahl, Christopher O'Callaghan, Judy Teale, Christine Wells
- Non-PIs with who have used resources to study related members of this paradigm group include: Roland Lang, Ulrich Maus, Gunnar Nilsson, Kenneth Rock
Progress toward understanding this GBP paradigm
This section documents what is currently known about MGL, its carbohydrate ligand(s), and how they interact to mediate cell communication. Further information about MGL can be found in its GBP Molecule Page in the CFG database.
Carbohydrate ligands
- mMGL1 binds Lewis X and Lewis A structures, whereas mMGL2 recognizes N-acetylgalactosamine (GalNAc) and galactose, including the O-linked Tn-antigen and TF-antigen [3][4][6]
- hMGL binds terminal α- and β-linked GalNAc residues on glycoproteins, glycolipids and bacterial LPS, including Tn antigen and GalNAcβ1-4GlcNAc-R (LDN) antigens [2][7][8][9][6].
- hMGL can also bind to STn when presented on a peptide or polyacrylamide backbone.
Cellular expression of GBP and ligands
MGL is expressed on dendritic cells and macrophages. [5][10]
The Tn ligand is expressed by many cancer cells especially breast cancers where it is expressed on more than 90% of breast carcinomas [11]. although STn is also expressed by carcinomas, especially colorectal and in 25-30% of breast cancers.
Biosynthesis of ligands
The Tn ligand can be expressed in cervical cancer due to mutations in Cosmc [12]. a molecular chaperone that is essential for the activity of the T synthase, the glycosyltransferase that catalyses the addition Gal to GalNAcαSer/Thr, forming the T antigen (Galβ1,3GalNAcαSer/Thr).
Although the vast majority of breast cancers express Tn there is no evidence of mutated Cosmc in these cancers therefore another mechanism for the expression of the Tn ligand must be active. Moreover the expression of STn in breast cancer is perfectly correlated with the turning on of the transcription of ST6GalNAc-I [13].
Structure
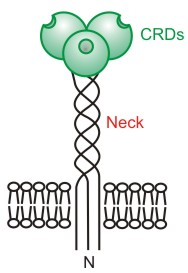
MGL is an oligomeric type II transmembrane protein. The CRD of the major subunit of the hepatic asialoglycoprotein receptor has been determined[14] and the structure of a galatose-binding mutant of mannose-binding protein provides experimental evidence for how galactose- and GalNAc-terminated ligands can bind to the receptor.[15]
Biological roles of GBP-ligand interaction
- MGL is a highly efficient internalization receptor [5][16]
- hMGL regulates T-cell receptor mediated signaling and T-cell dependent cytokine responses [10]
- mMGL1 promotes adipose tissue inflammation and insulin resistance [17]
- mMGL2 promotes enhances both MHC class II and class I presentation antigen in dendritic cells (DCs) [18]
CFG resources used in investigations
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the CFG database search results for MGL.
Glycan profiling
Glycogene microarray
Probes for the single human MGL and both mouse MGLs have been included in all versions of the CFG glycogene chip.
Knockout mouse lines
Mice lacking MGL-1[19] were distributed by the CFG and the phenotype was analyzed. Mice lacking MGL-2 have also been described.[20] ES cells for an MGL-1/2 double knockout, generated in Core F, are available from MMRRC.
Glycan array
The glycan-binding specificity of human and mouse versions of MGL have been analyzed by glycan array screening [7][4]. See all glycan array results for MGL here. See glycan array results for these related GBPs: mannose receptor, mincle, macrophage C-type lectin (MCL), and dectin-1.
Related GBPs
Other C-type lectins on macrophages include the mannose receptor (CFG data), mincle [21] (CFG data), macrophage C- type lectin (MCL) (CFG data), and dectin-1 (CFG data).
References
- ↑ Kawasaki T, Ii M, Kozutsumi Y and Yamashina I. 1986. Isolation and characterization of a receptor lectin specific for galactose/N-acetylgalactosamine from macrophages. Carbohydr Res. 151:197-206
- ↑ 2.0 2.1 Suzuki N, Yamamoto K, Toyoshima S, Osawa T and Irimura T. 1996. Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J Immunol. 156:128-135
- ↑ 3.0 3.1 Tsuiji M, Fujimori M, Ohashi Y, Higashi N, Onami TM, Hedrick SM and Irimura T. 2002. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J Biol Chem. 277:28892-28901
- ↑ 4.0 4.1 4.2 Singh SK, Streng-Ouwehand I, Litjens M, Weelij DR, Garca-Vallejo JJ, van Vliet SJ, Saeland E, van Kooyk Y. 2009. Characterization of murine MGL1 and MGL2 C-type lectins: distinct glycan specificities and tumor binding properties. Mol Immunol 46: 1240-1249
- ↑ 5.0 5.1 5.2 Higashi N, Fujioka K, Denda-Nagai K, Hashimoto S, Nagai S, Sato T, Fujita Y, Morikawa A, Tsuiji M, Miyata-Takeuchi M, Sano Y, Suzuki N, Yamamoto K, Matsushima K and Irimura T. 2002. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem. 277:20686-20693
- ↑ 6.0 6.1 Napoletano C, Rughetti A, Tarp M.P.A, Coleman J Bennett,E.P, Picco G, Sale P, Denda-Hagai K, Irimura T, Mandel U, Clausen H, Frati L, Taylor-Papadimitriou J, Burchell J, Nuti M. Tumour associated Tn-MUC1 glycoform is internalised througfh the macrophage galactose C-type lectin and delivered to the HLA class I and Class II compartments in dendritic cells. Cancer Research, 2007, 67(17): 8358-8367
- ↑ 7.0 7.1 van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, Geijtenbeek TB, Blixt O, Alvarez R, van Die I and van Kooyk Y. 2005. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 17:661-669
- ↑ van Sorge NM, Bleumink NM, van Vliet SJ, Saeland E, van der Pol WL, van Kooyk Y and van Putten JP. 2009. N-glycosylated proteins and distinct lipooligosaccharide glycoforms of Campylobacter jejuni target the human C-type lectin receptor MGL. Cell Microbiol. 11:1768-1781
- ↑ Saeland E, van Vliet SJ, Backstrom M, van den Berg VC, Geijtenbeek TB, Meijer GA and van Kooyk Y. 2007. The C-type lectin mgl expressed by dendritic cells detects glycan changes on Muc1 in colon carcinoma. Cancer Immunol Immunother. 56:1225-1236
- ↑ 10.0 10.1 van Vliet SJ, Gringhuis SI, Geijtenbeek TB and van Kooyk Y. 2006. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 7:1200-1208
- ↑ Sørensen AL, “et al”. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. “Glycobiology” 16, 96-107 (2006)
- ↑ Ju T, “et al”. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc “Cancer Research” 68, 1636-1646 (2008)
- ↑ Sewell R, “et al”. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. “J Biol Chem” 281, 3586-3594 (2006)
- ↑ Meier, M, Bider, MD, Malashkevich, VN, Spiess, M and Burkhard, P. 2000. Crystal structure of the carbohydrate recognition domain of the H1 subunit of the asialoglycoprotein receptor. J Mol Biol 300:857–865
- ↑ Kolatkar, AR, Leung, AK, Isecke, R, Brossmer, R, Drickamer, K and Weis, WI. 1998. Mechanism of N-acetylgalactosamine binding to a C-type animal lectin carbohydrate-recognition domain. J Biol Chem 273:19502-19508
- ↑ Valladeau J, Duvert-Frances V, Pin JJ, Kleijmeer MJ, Ait-Yahia S, Ravel O, Vincent C, Vega F, Jr., Helms A, Gorman D, Zurawski SM, Zurawski G, Ford J and Saeland S. 2001. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J Immunol. 167:5767-5774
- ↑ Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. 2009. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med 206: 3143-56
- ↑ Singh SK, Streng-Ouwehand I, Litjens M, Kalay H, Saeland E, Van Kooyk Y. 2010. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int. J. Cancer, in press
- ↑ Onami, TM, Lin, M-Y, Page, DM, Shirley A. Reynolds, SA, Katayama, CD, Marth, JD, Irimura, T, Varki, A, Varki, N and Hedrick SM (2002) Generation of mice deficient for dacrophage galactose- and N-acetylgalactosamine-specific lectin: limited role in lymphoid and erythroid homeostasis and evidence for multiple lectins. Mol. Cell. Biol. 22, 5173-5181
- ↑ Denda-Nagai, K, Aida, S, Saba, K, Suzuki, K, Moriyama, S, Oo-puthinan, S, Tsuiji, M, Morikawa, A, Kumamoto, Y, Sugiura, D, Kudo, A, Akimoto, Y, Kawakami, H, Bovin NV, and Irimura, T (2010) Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J. Biol. Chem. 285, 19193–19204
- ↑ Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, Schroder K, Ma B, Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180: 7404-7413
Acknowledgements
The CFG is grateful to the following PIs for their contributions to this wiki page: Kurt Drickamer, Joyce Taylor-Papadimitriou, Yvette van Kooyk, Irma van Die