Difference between revisions of "PA-IIL"
Anne Imberty (talk | contribs) |
|||
| (20 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | PA-IIL (Pseudomonas lectin II, LecB) is a cytoplasmic lectin produced in ''Pseudomonas aruginosa'' under the control of quorum sensing. It is a virulence factor involved in binding of human tissues such as epithelial lung of cystic fibrosis patients<ref>Imberty, A., Wimmerova, M., Mitchell, E. P. & Gilboa-Garber, N. (2004). Structures of the lectins from Pseudomonas aeruginosa: Insights into molecular basis for host glycan recognition. Microb. Infect. 6, 222-229.</ref>. The role of PA-IIL in pathogenesis has been highlighted, since interfering with PA-IIL binding site reduces the mortality of ''P. aeruginosa'' induced-pneumonia in a murine model<ref>Chemani, C., Imberty, A., de Bentzman, S., Pierre, P., Wimmerová, M., Guery, B. P. & Faure, K. (2009). Role of LecA and LecB lectins in Pseudomonas aeruginosa induced lung injury and effect of carbohydrates ligands. Infect. Immun. 77, 2065-2075.</ref>. The paradigm is unique among glycan-binding proteins (GBPs) in that it contains two | + | PA-IIL (Pseudomonas lectin II, LecB) is a cytoplasmic lectin produced in ''Pseudomonas aruginosa'' under the control of quorum sensing. It is a virulence factor involved in binding of human tissues such as epithelial lung of cystic fibrosis patients<ref name="Imberty 2004">Imberty, A., Wimmerova, M., Mitchell, E. P. & Gilboa-Garber, N. (2004). Structures of the lectins from Pseudomonas aeruginosa: Insights into molecular basis for host glycan recognition. Microb. Infect. 6, 222-229.</ref>. The role of PA-IIL in pathogenesis has been highlighted, since interfering with PA-IIL binding site reduces the mortality of ''P. aeruginosa'' induced-pneumonia in a murine model<ref name="Chemani 2009">Chemani, C., Imberty, A., de Bentzman, S., Pierre, P., Wimmerová, M., Guery, B. P. & Faure, K. (2009). Role of LecA and LecB lectins in Pseudomonas aeruginosa induced lung injury and effect of carbohydrates ligands. Infect. Immun. 77, 2065-2075.</ref>. The paradigm is unique among glycan-binding proteins (GBPs) in that it contains two cationic ions in the carbohydrate binding site that result in unusual high affinity for the carbohydrate target<ref>Loris, R., Tielker, D., Jaeger, K.-E. & Wyns, L. (2003). Structural basis of carbohydrate recognition by the lectin LecB from Pseudomonas aeruginosa. J. Mol. Biol. 331, 861-870.</ref><ref name="test 1">Mitchell, E., Houles, C., Sudakevitz, D., Wimmerova, M., Gautier, C., Pérez, S., Wu, A. M., Gilboa-Garber, N. & Imberty, A. (2002). Structural basis for oligosaccharide-mediated adhesion of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Nature Struct. Biol. 9, 918-921.</ref><ref>Mitchell, E. P., Sabin, C., Šnajdrová, L., Pokorná, M., Perret, S., Gautier, C., Hofr, C., Gilboa-Garber, N., Koča, J., Wimmerová, M. & Imberty, A. (2005). High affinity fucose binding of Pseudomonas aeruginosa lectin PA-IIL: 1.0 Å resolution crystal structure of the complex combined with thermodynamics and computational chemistry approaches. Proteins: Struct. Funct. Bioinfo. 58, 735-748.</ref>. The preferred glycan ligand of PA-IIL is the trisaccharide Lewis a<ref name="test 2">Perret, S., Sabin, C., Dumon, C., Pokorná, M., Gautier, C., Galanina, O., Ilia, S., Bovin, N., Nicaise, M., Desmadril, M., Gilboa-Garber, N., Wimmerova, M., Mitchell, E. P. & Imberty, A. (2005). Structural basis for the interaction between human milk oligosaccharides and the bacterial lectin PA-IIL of Pseudomonas aeruginosa. Biochem. J. 389, 325-332.</ref>. PA-IIL-like lectins have been characterized in other opportunistic bacteria such as ''Ralstonia solanacearum''<ref>Sudakevitz, D., Kostlanova, N., Blatman-Jan, G., Mitchell, E. P., Lerrer, B., Wimmerova, M., Katcof, f. D. J., Imberty, A. & Gilboa-Garber, N. (2004). A new Ralstonia solanacearum high affinity mannose-binding lectin RS-IIL structurally resembling the Pseudomonas aeruginosa fucose-specific lectin PA-IIL. Mol. Microbiol. 52, 691-700.</ref>, ''Chromobacterium violaceum''<ref name="Pokorna 11">Pokorná, M., Cioci, G., Perret, S., Rebuffet, E., Kostlánová, N., Adam, J., Gilboa-Garber, N., Mitchell, E. P., Imberty, A. & Wimmerová, M. (2006). Unusual entropy driven affinity of Chromobacterium violaceum lectin CV-IIL towards fucose and mannose. Biochemistry 45, 7501-7510.</ref>, and ''Burkholderia cenocepacia''<ref>Lameignere, E., Malinovská, L., Sláviková, M., Duchaud, E., Mitchell, E. P., Varrot, A., Šedo, O., Imberty, A. & Wimmerová, M. (2008). Structural basis for mannose recognition by a lectin from opportunistic bacteria Burkholderia cenocepacia. Biochem. J. 411, 307-318.</ref><ref>Lameignere, E., Shiao, T. C., Roy, R., Wimmerová, M., Dubreuil, F., Varrot, A. & Imberty, A. (2010). Structural basis of the affinity for oligomannosides and analogs displayed by BC2L-A, a Burkholderia cenocepacia soluble lectin. Glycobiology 20, 87-98.</ref>, albeit with some variations in the fine specificity. Starting from the results obtained with the help of CFG tools, major efforts are devoided in collaboration with carbohydrate chemists in order to design anti-bacterial new glyco-derived compounds that bind to PA-IIL with high affinity<ref>Imberty, A., Chabre, Y. M. & Roy, R. (2008). Glycomimetics and glycodendrimers as high affinity microbial antiadhesins. Chemistry 14, 7490-7499.</ref>. |
== CFG Participating Investigators contributing to the understanding of this paradigm == | == CFG Participating Investigators contributing to the understanding of this paradigm == | ||
| Line 5: | Line 5: | ||
== Progress toward understanding this GBP paradigm == | == Progress toward understanding this GBP paradigm == | ||
| − | + | This section documents what is currently known about PA-IIL, its carbohydrate ligand(s), and how they interact to mediate cell communication. | |
=== Carbohydrate ligands === | === Carbohydrate ligands === | ||
| − | The high affinity ligand for PA-IIL has been deduced from glycan microarray screening on the CFG microarray[http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_GLYCAN_v3_221_02142006#] to be Galβ1-4(Fucα1-4)GlcNAc [Lewis a]<ref>Marotte K, Sabin C, Preville C, Moume-Pymbock M, Wimmerova M, Mitchell EP, Imberty A, Roy R. X-ray Structures and | + | PA-IIL is a fucose-specific lectin that also recognises mannose residues.<br> |
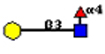
| + | The high affinity ligand for PA-IIL has been deduced from glycan microarray screening on the CFG microarray[http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_GLYCAN_v3_221_02142006#] to be Galβ1-4(Fucα1-4)GlcNAc [Lewis a] <ref name="test 1"></ref><ref name="test 2"></ref><ref>Marotte K, Sabin C, Preville C, Moume-Pymbock M, Wimmerova M, Mitchell EP, Imberty A, Roy R. (2008) X-ray Structures and thermodynamics of the interaction of PA-IIL from Pseudomonas aeruginosa with disaccharide derivatives. ChemMedChem 10,1328-1338.</ref> | ||
[[File:lewisa.jpg]] | [[File:lewisa.jpg]] | ||
| − | |||
| − | |||
| + | === Cellular expression of GBP and ligands === | ||
| + | PA-IIL is produced in ''Pseudomonas aruginosa,'' and binds ligands in human tissues, including epithelial lung. | ||
| + | <br> | ||
| + | === Biosynthesis of ligands === | ||
| + | Lewis a synthesis requires the addition of α1-4 fucose to the sub-terminal GlcNAc residue on a type 1 chain. Addition of the α1-4 fucose is catalyzed exclusively by fucosyltransferase FUT3 [http://www.functionalglycomics.org/glycomics/molecule/jsp/glycoEnzyme/viewGlycoEnzyme.jsp?gbpId=gt_hum_600&sideMenu=true&pageType=general]. | ||
<br> | <br> | ||
| + | |||
=== Structure === | === Structure === | ||
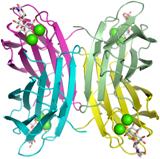
| + | PA-IIL is a tetrameric lectin. Each monomer consists of a β-sandwich and contains two calcium ions that interact directly with the carbohydrate ligand. Crystal structures have been determined for PA-IIL alone, and its complex with fucose, with related monosaccharides, and with complex fucosylated oligosaccharides. | ||
| + | [[File:pa2ls.jpg]] | ||
<br> | <br> | ||
| + | Information on crystal structures of PA-IIL and links to PDB are available at PA-IIL page of [http://www.cermav.cnrs.fr/cgi-bin/lectines/menu.cgi?Bacterial%20lectins+2-CA%20b-sandwich+Pseudomonas%20PA-IIL 3D-lectin database] | ||
| + | |||
=== Biological roles of GBP-ligand interaction === | === Biological roles of GBP-ligand interaction === | ||
| + | PA-IIL, a virulence factor that binds human tissues such as epithelial lung of cystic fibrosis patients<ref name="Imberty 2004"/>, has roles in pathogenesis <ref name="Chemani 2009"/>. | ||
| + | <br> | ||
| − | |||
== CFG resources used in investigations == | == CFG resources used in investigations == | ||
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=PA-IIL&maxresults=20 CFG database search results for PA-IIL]. | The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=PA-IIL&maxresults=20 CFG database search results for PA-IIL]. | ||
| Line 30: | Line 40: | ||
<br> | <br> | ||
=== Glycogene microarray === | === Glycogene microarray === | ||
| + | PA-IIL is not represented on the CFG microarrays, which only contain probes for mouse and human glycogenes. | ||
| + | <br> | ||
| + | === Knockout mouse lines === | ||
| + | Not applicable. | ||
<br> | <br> | ||
| − | |||
| − | |||
=== Glycan array === | === Glycan array === | ||
| − | The specificity of PA-IIl and related proteins was determined through glycan array analysis ([http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_PA_v2_230_02102006 | + | The specificity of PA-IIl and related proteins was determined through glycan array analysis (click [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_PA_v2_230_02102006 here] and [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_GLYCAN_v3_221_02142006# here]). |
== Related GBPs == | == Related GBPs == | ||
| − | CV-IIL, RS-IIL, BC2L-A, BC2L-B, | + | CV-IIL [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=CV-IIL&maxresults=20 (CFG data)], RS-IIL [http://www.functionalglycomics.org/glycomics/search/jsp/result.jsp?query=RS-IIL&cat=coreh (CFG data)], BC2L-A, BC2L-B [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=BC2L-B&maxresults=20 (CFG data)], BC2L-C [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=BC2L-C&maxresults=20 (CFG data)]. |
== References == | == References == | ||
Latest revision as of 09:29, 9 October 2011
PA-IIL (Pseudomonas lectin II, LecB) is a cytoplasmic lectin produced in Pseudomonas aruginosa under the control of quorum sensing. It is a virulence factor involved in binding of human tissues such as epithelial lung of cystic fibrosis patients[1]. The role of PA-IIL in pathogenesis has been highlighted, since interfering with PA-IIL binding site reduces the mortality of P. aeruginosa induced-pneumonia in a murine model[2]. The paradigm is unique among glycan-binding proteins (GBPs) in that it contains two cationic ions in the carbohydrate binding site that result in unusual high affinity for the carbohydrate target[3][4][5]. The preferred glycan ligand of PA-IIL is the trisaccharide Lewis a[6]. PA-IIL-like lectins have been characterized in other opportunistic bacteria such as Ralstonia solanacearum[7], Chromobacterium violaceum[8], and Burkholderia cenocepacia[9][10], albeit with some variations in the fine specificity. Starting from the results obtained with the help of CFG tools, major efforts are devoided in collaboration with carbohydrate chemists in order to design anti-bacterial new glyco-derived compounds that bind to PA-IIL with high affinity[11].
CFG Participating Investigators contributing to the understanding of this paradigm
CFG Participating Investigators (PIs) have made major contribution to the understanding of the structure/specificity relationship of PA-IIL and PA-IIL-like proteins. These include: Anne Imberty, Remy Loris, Michaela Wimmerova
Progress toward understanding this GBP paradigm
This section documents what is currently known about PA-IIL, its carbohydrate ligand(s), and how they interact to mediate cell communication.
Carbohydrate ligands
PA-IIL is a fucose-specific lectin that also recognises mannose residues.
The high affinity ligand for PA-IIL has been deduced from glycan microarray screening on the CFG microarray[1] to be Galβ1-4(Fucα1-4)GlcNAc [Lewis a] [4][6][12]
Cellular expression of GBP and ligands
PA-IIL is produced in Pseudomonas aruginosa, and binds ligands in human tissues, including epithelial lung.
Biosynthesis of ligands
Lewis a synthesis requires the addition of α1-4 fucose to the sub-terminal GlcNAc residue on a type 1 chain. Addition of the α1-4 fucose is catalyzed exclusively by fucosyltransferase FUT3 [2].
Structure
PA-IIL is a tetrameric lectin. Each monomer consists of a β-sandwich and contains two calcium ions that interact directly with the carbohydrate ligand. Crystal structures have been determined for PA-IIL alone, and its complex with fucose, with related monosaccharides, and with complex fucosylated oligosaccharides.
Information on crystal structures of PA-IIL and links to PDB are available at PA-IIL page of 3D-lectin database
Biological roles of GBP-ligand interaction
PA-IIL, a virulence factor that binds human tissues such as epithelial lung of cystic fibrosis patients[1], has roles in pathogenesis [2].
CFG resources used in investigations
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the CFG database search results for PA-IIL.
Glycan profiling
Glycogene microarray
PA-IIL is not represented on the CFG microarrays, which only contain probes for mouse and human glycogenes.
Knockout mouse lines
Not applicable.
Glycan array
The specificity of PA-IIl and related proteins was determined through glycan array analysis (click here and here).
Related GBPs
CV-IIL (CFG data), RS-IIL (CFG data), BC2L-A, BC2L-B (CFG data), BC2L-C (CFG data).
References
- ↑ 1.0 1.1 Imberty, A., Wimmerova, M., Mitchell, E. P. & Gilboa-Garber, N. (2004). Structures of the lectins from Pseudomonas aeruginosa: Insights into molecular basis for host glycan recognition. Microb. Infect. 6, 222-229.
- ↑ 2.0 2.1 Chemani, C., Imberty, A., de Bentzman, S., Pierre, P., Wimmerová, M., Guery, B. P. & Faure, K. (2009). Role of LecA and LecB lectins in Pseudomonas aeruginosa induced lung injury and effect of carbohydrates ligands. Infect. Immun. 77, 2065-2075.
- ↑ Loris, R., Tielker, D., Jaeger, K.-E. & Wyns, L. (2003). Structural basis of carbohydrate recognition by the lectin LecB from Pseudomonas aeruginosa. J. Mol. Biol. 331, 861-870.
- ↑ 4.0 4.1 Mitchell, E., Houles, C., Sudakevitz, D., Wimmerova, M., Gautier, C., Pérez, S., Wu, A. M., Gilboa-Garber, N. & Imberty, A. (2002). Structural basis for oligosaccharide-mediated adhesion of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Nature Struct. Biol. 9, 918-921.
- ↑ Mitchell, E. P., Sabin, C., Šnajdrová, L., Pokorná, M., Perret, S., Gautier, C., Hofr, C., Gilboa-Garber, N., Koča, J., Wimmerová, M. & Imberty, A. (2005). High affinity fucose binding of Pseudomonas aeruginosa lectin PA-IIL: 1.0 Å resolution crystal structure of the complex combined with thermodynamics and computational chemistry approaches. Proteins: Struct. Funct. Bioinfo. 58, 735-748.
- ↑ 6.0 6.1 Perret, S., Sabin, C., Dumon, C., Pokorná, M., Gautier, C., Galanina, O., Ilia, S., Bovin, N., Nicaise, M., Desmadril, M., Gilboa-Garber, N., Wimmerova, M., Mitchell, E. P. & Imberty, A. (2005). Structural basis for the interaction between human milk oligosaccharides and the bacterial lectin PA-IIL of Pseudomonas aeruginosa. Biochem. J. 389, 325-332.
- ↑ Sudakevitz, D., Kostlanova, N., Blatman-Jan, G., Mitchell, E. P., Lerrer, B., Wimmerova, M., Katcof, f. D. J., Imberty, A. & Gilboa-Garber, N. (2004). A new Ralstonia solanacearum high affinity mannose-binding lectin RS-IIL structurally resembling the Pseudomonas aeruginosa fucose-specific lectin PA-IIL. Mol. Microbiol. 52, 691-700.
- ↑ Pokorná, M., Cioci, G., Perret, S., Rebuffet, E., Kostlánová, N., Adam, J., Gilboa-Garber, N., Mitchell, E. P., Imberty, A. & Wimmerová, M. (2006). Unusual entropy driven affinity of Chromobacterium violaceum lectin CV-IIL towards fucose and mannose. Biochemistry 45, 7501-7510.
- ↑ Lameignere, E., Malinovská, L., Sláviková, M., Duchaud, E., Mitchell, E. P., Varrot, A., Šedo, O., Imberty, A. & Wimmerová, M. (2008). Structural basis for mannose recognition by a lectin from opportunistic bacteria Burkholderia cenocepacia. Biochem. J. 411, 307-318.
- ↑ Lameignere, E., Shiao, T. C., Roy, R., Wimmerová, M., Dubreuil, F., Varrot, A. & Imberty, A. (2010). Structural basis of the affinity for oligomannosides and analogs displayed by BC2L-A, a Burkholderia cenocepacia soluble lectin. Glycobiology 20, 87-98.
- ↑ Imberty, A., Chabre, Y. M. & Roy, R. (2008). Glycomimetics and glycodendrimers as high affinity microbial antiadhesins. Chemistry 14, 7490-7499.
- ↑ Marotte K, Sabin C, Preville C, Moume-Pymbock M, Wimmerova M, Mitchell EP, Imberty A, Roy R. (2008) X-ray Structures and thermodynamics of the interaction of PA-IIL from Pseudomonas aeruginosa with disaccharide derivatives. ChemMedChem 10,1328-1338.
Acknowledgements
The CFG is grateful to the following PIs for their contributions to this wiki page: Anne Imberty and Micha Wimmerova