Difference between revisions of "F17G/GafD"
| (40 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | The F17-G (GafD) adhesin at the tip of flexible F17 fimbriae of enterotoxigenic ''Escherichia coli'' mediates binding to N-acetyl-β-D-glucosamine-presenting receptors on the microvilli of the intestinal epithelium of ruminants, leading to diarrhea or septicaemia. F17-G belong to two-domain adhesins (TDA)s consisting of a pilin domain and a lectin domain, both having an Ig-fold joined via a short interdomain linker<ref>Buts, L., Bouckaert, J., De Gents, E., Loris, R., Oscarson, S., Lahmann, M., Messens, J., Brosens, E., Wyns, L. & De Greve, H. (2003). The fimbrial adhesin F17-G of enterotoxigenic Escherichia coli has an immunoglobulin-like lectin domain that binds N-acetylglucosamine. Mol. Microb. 49, 705-715.</ref><ref>Merckel, M. C., Tanskanen, J., Edelman, S., Westerlund-Wilkström, B., Korhonen, T. K. & Goldman, A. (2003). The structural basis of receptor-binding by Escherichia coli associaed with diarrhea and septicemia. J. Mol. Biol. 331, 897-905.</ref>. Related adhesins have been characterized in enteropathogenic ''E. coli'' ( FedF on F18 fimbriae<ref>Coddens, A., Diswall, M., Angstrom, J., Breimer, M. E., Goddeeris, B., Cox, E. & Teneberg, S. (2009). Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J Biol Chem 284, 9713-26.</ref> and CfaE on CFA/I pili<ref>Poole, S. T., McVeigh, A. L., Anantha, R. P., Lee, L. H., Akay, Y. M., Pontzer, E. A., Scott, D. A., Bullitt, E. & Savarino, S. J. (2007). Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63, 1372-84.</ref>) ) and uropathogenic ones (FimH on type 1 fimbriae<ref>Bouckaert, J., Berglund, J., Schembri, M., De Gents, E., Cools, L., Wuhrer, M., Hung, C.-S., Pinkner, J., Slättegard, R., Savialov, A., Choudhury, D., Langermann, S., Hultgren, S. J., Wyns, L., Klemm, P., Oscarson, S., Knight, S. D. & De Greve, H. (2005). Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microb. 55, 441-455.</ref> and PapG on P-pili<ref>Dodson, K. W., Pinkner, J. S., Rose, T., Magnusson, G., Hultgren, S. J. & Waksman, G. (2001). Structural basis of the interaction of the pyelonephritic E. coli adhesin to ist human kideny receptor. Cell 105, 733-743.</ref>). Fimbrial adhesins from other organisms, such as CupB6 from ''Pseudomonas aeruginosa'' are also investigated. All share the immunoglobulin-like fold of the two structural components, despite lack of any sequence identity and diversity in carbohydrate specificity and binding site, and the corresponding pili are assembled by the chaperone-usher pathway<ref>De Greve, H., Wyns, L. & Bouckaert, J. (2007). Combining sites of bacterial fimbriae. Curr Opin Struct Biol 17, 506-12.</ref><ref>Sauer, F. G., Barnhart, M., Choudhury, D., Knight, S. D., Waksman, G. & Hultgren, S. J. (2000). Chaperone-assisted pilus assembly and bacterial attachment. Curr Opin Struct Biol 10, 548-56.</ref>. The paradigm is unique among TAD for his specificity toward GlcNAc. The binding site is located laterally and not at the tip of the pili, therefore the long and flexible F17 fimbriae could intrude between the microvilli of the epithelium, with the binding site of the lectin domain interacting laterally with GlcNAc-containing receptors. Five naturally occurring variants, differing in 1-18 amino acids of the adhesion domain have been identified<ref>De Kerpel, M., Van Molle, I., Brys, L., Wyns, L., De Greve, H. & Bouckaert, J. (2006). N-terminal truncation enables crystallization of the receptor-binding domain of the FedF bacterial adhesin. Acta Crystallogr Sect F Struct Biol Cryst Commun 62, 1278-82.</ref>. | + | The F17-G (GafD) adhesin at the tip of flexible F17 fimbriae of enterotoxigenic ''Escherichia coli'' mediates binding to N-acetyl-β-D-glucosamine-presenting receptors on the microvilli of the intestinal epithelium of ruminants, leading to diarrhea or septicaemia. F17-G belong to two-domain adhesins (TDA)s consisting of a pilin domain and a lectin domain, both having an Ig-fold joined via a short interdomain linker |
| + | <ref name=" Buts, L2003"> | ||
| + | Buts, L., Bouckaert, J., De Gents, E., Loris, R., Oscarson, S., Lahmann, M., Messens, J., Brosens, E., Wyns, L. & De Greve, H. (2003). The fimbrial adhesin F17-G of enterotoxigenic Escherichia coli has an immunoglobulin-like lectin domain that binds N-acetylglucosamine. Mol. Microb. 49, 705-715.</ref><ref>Merckel, M. C., Tanskanen, J., Edelman, S., Westerlund-Wilkström, B., Korhonen, T. K. & Goldman, A. (2003). The structural basis of receptor-binding by Escherichia coli associaed with diarrhea and septicemia. J. Mol. Biol. 331, 897-905.</ref>. Related adhesins have been characterized in enteropathogenic ''E. coli'' ( FedF on F18 fimbriae<ref>Coddens, A., Diswall, M., Angstrom, J., Breimer, M. E., Goddeeris, B., Cox, E. & Teneberg, S. (2009). Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J Biol Chem 284, 9713-26.</ref> and CfaE on CFA/I pili<ref>Poole, S. T., McVeigh, A. L., Anantha, R. P., Lee, L. H., Akay, Y. M., Pontzer, E. A., Scott, D. A., Bullitt, E. & Savarino, S. J. (2007). Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63, 1372-84.</ref>) ) and uropathogenic ones (FimH on type 1 fimbriae<ref>Bouckaert, J., Berglund, J., Schembri, M., De Gents, E., Cools, L., Wuhrer, M., Hung, C.-S., Pinkner, J., Slättegard, R., Savialov, A., Choudhury, D., Langermann, S., Hultgren, S. J., Wyns, L., Klemm, P., Oscarson, S., Knight, S. D. & De Greve, H. (2005). Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microb. 55, 441-455.</ref> and PapG on P-pili<ref>Dodson, K. W., Pinkner, J. S., Rose, T., Magnusson, G., Hultgren, S. J. & Waksman, G. (2001). Structural basis of the interaction of the pyelonephritic E. coli adhesin to ist human kideny receptor. Cell 105, 733-743.</ref>). Fimbrial adhesins from other organisms, such as CupB6 from ''Pseudomonas aeruginosa'' are also investigated. All share the immunoglobulin-like fold of the two structural components, despite lack of any sequence identity and diversity in carbohydrate specificity and binding site, and the corresponding pili are assembled by the chaperone-usher pathway<ref>De Greve, H., Wyns, L. & Bouckaert, J. (2007). Combining sites of bacterial fimbriae. Curr Opin Struct Biol 17, 506-12.</ref><ref>Sauer, F. G., Barnhart, M., Choudhury, D., Knight, S. D., Waksman, G. & Hultgren, S. J. (2000). Chaperone-assisted pilus assembly and bacterial attachment. Curr Opin Struct Biol 10, 548-56.</ref>. The paradigm is unique among TAD for his specificity toward GlcNAc. The binding site is located laterally and not at the tip of the pili, therefore the long and flexible F17 fimbriae could intrude between the microvilli of the epithelium, with the binding site of the lectin domain interacting laterally with GlcNAc-containing receptors. Five naturally occurring variants, differing in 1-18 amino acids of the adhesion domain have been identified<ref>De Kerpel, M., Van Molle, I., Brys, L., Wyns, L., De Greve, H. & Bouckaert, J. (2006). N-terminal truncation enables crystallization of the receptor-binding domain of the FedF bacterial adhesin. Acta Crystallogr Sect F Struct Biol Cryst Commun 62, 1278-82.</ref>. | ||
== CFG Participating Investigators contributing to the understanding of this paradigm == | == CFG Participating Investigators contributing to the understanding of this paradigm == | ||
| Line 5: | Line 7: | ||
== Progress toward understanding this GBP paradigm == | == Progress toward understanding this GBP paradigm == | ||
| − | + | This section documents what is currently known about F17G/GafD, its carbohydrate ligand(s), and how they interact to mediate cell communication. | |
=== Carbohydrate ligands === | === Carbohydrate ligands === | ||
| − | + | The F17G adhesin is most specific for the disaccharide GlcNAcb1,3Gal that can be recognised as a terminal or internal sequence in bovine glycophorin <ref>Mouricout, M., Milhavet, M., Durié, C., Grange, P. Characterization of glycoprotein glycan receptors for Escherichia coli F17 fimbrial lectin, Microb. Pathog. (1995) 18, 297-306</ref>: | |
| + | <br> | ||
| + | [[File:carbSynthe_0691_D000.jpg]] | ||
| + | <br> | ||
| + | The branched form gives a closely similar fluorescent signal: | ||
| + | <br> | ||
| + | [[File:carbSynthe_0897_D000.jpg]] | ||
| + | <br> | ||
| + | The presence of the b1,3 linkage of N-acetyl glucosamine to galactose enhances the affinity for F17G at least 2-fold, compared to the monosaccharide N-acetyl glucosamine, as validated using surface plasmon resonance measurements. Second best binders are the b1,4 and b1,6 galactose linked disaccharides, whereas chitobiose, that is also a characterized inhibitor of F17G-mediated bacterial adhesion, is clearly lagging behind. F17G can thus be ranked under glycan binding proteins that display high selectivity. | ||
| − | === Cellular expression === | + | === Cellular expression of GBP and ligands === |
| + | F17G adhesins are expressed on enterotoxigenic E. coli infecting neonatal lambs, calves, and goat kids. | ||
| + | The F17G ligand GlcNAcb1,3Gal occurs universally but mostly internally in the sequence of poly-lactosaminyl glycans and blood group antigens. | ||
| + | These glycan structures are widely expressed on mamalian cell surfaces. | ||
<br> | <br> | ||
| + | F17-fimbriated E. coli predominantly colonize neonatal animals, but also are a major causal agent (55%) of mastitis in bovines <ref>Lipman, L.J.A., de Nijs A., Gaastra W.. Isolation and identification of fimbriae and toxin production by Escherichia coli strains from cows with clinical mastitis, Vet. Microbiology 47 (1995) p. 1-7 </ref>. Congruent with the glycans recognized by F17G on the printed array versions 2.1 and 4.1, the N-acetyl glucosamine residue of GlcNAcb1,3Gal may be unsubstituted at the early life stage of calves, that are at the same time protected from bacterial infections by glycans secreted in the cow's milk. | ||
| + | <br> | ||
| + | |||
| + | === Biosynthesis of ligands === | ||
| + | The target sequence GlcNAcβ1-3Gal appears in O-linked glycans, where it is synthesized by [http://www.functionalglycomics.org/glycomics/molecule/jsp/glycoEnzyme/viewGlycoEnzyme.jsp?gbpId=gt_hum_536&sideMenu=true&pageType=general UDP-GlcNAc:βGalβ1-3 GlcNAc transferase 3]. Other enzymes that can synthesize this linkage on N- and O-linked glycans include | ||
| + | [http://www.functionalglycomics.org/glycomics/molecule/jsp/glycoEnzyme/viewGlycoEnzyme.jsp?gbpId=gt_hum_537&sideMenu=true&pageType=general UDP-GlcNAc:βGal β1-3 GlcNAc transferase 4], | ||
| + | [http://www.functionalglycomics.org/glycomics/molecule/jsp/glycoEnzyme/viewGlycoEnzyme.jsp?gbpId=gt_hum_538&sideMenu=true&pageType=general UDP-GlcNAc:βGal β1-3 GlcNAc transferase 5], | ||
| + | [http://www.functionalglycomics.org/glycomics/molecule/jsp/glycoEnzyme/viewGlycoEnzyme.jsp?gbpId=gt_hum_547&sideMenu=true&pageType=general UDP-GlcNAc:βGal β1-3 GlcNAc transferase 6], and | ||
| + | [http://www.functionalglycomics.org/glycomics/molecule/jsp/glycoEnzyme/viewGlycoEnzyme.jsp?gbpId=gt_hum_562&sideMenu=true&pageType=general UDP-GlcNAc:βGal β1-3 GlcNAc transferase 7]. | ||
| + | <br> | ||
| + | |||
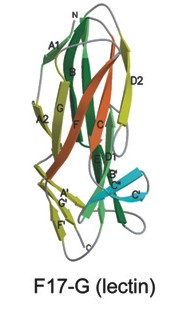
=== Structure === | === Structure === | ||
| + | The F17G adhesin is a two-domain adhesin (TDA) located at the F17 fimbrial tip. The determination of the crystal structure of the F17G lectin domain led to the discovery of the variable immunoglobulin-like structure | ||
| + | as a paradigm for bacterial fimbrial TDAs <ref name=" Buts, L2003"> | ||
| + | </ref>. F17G has a shallow groove for carbohydrate recognition on its flank. | ||
| − | < > | + | <br> |
| + | [[File:F17G_Igfold_v3.jpg]] | ||
| + | <br> | ||
=== Biological roles of GBP-ligand interaction === | === Biological roles of GBP-ligand interaction === | ||
| − | + | The F17G fimbrial lectin enhances intestinal colonization in the early life of ruminants. The long and flexible F17 fimbriae can penetrate deep between intestinal microvilli, where the fimbrial tip adhesin finds its glycan receptors. The subsequent secretion of heat stable and heat labile toxins can lead to severe diarrhea. | |
| + | |||
== CFG resources used in investigations == | == CFG resources used in investigations == | ||
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the CFG database search results for [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=fimbriae&maxresults=20 fimbriae] and [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=pili&maxresults=20 pili]. | The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the CFG database search results for [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=fimbriae&maxresults=20 fimbriae] and [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=pili&maxresults=20 pili]. | ||
| Line 27: | Line 57: | ||
<br> | <br> | ||
=== Glycogene microarray === | === Glycogene microarray === | ||
| + | F17G/GafD is not represented on the CFG microarrays, which only contain probes for mouse and human glycogenes. | ||
| + | <br> | ||
| + | === Knockout mouse lines === | ||
| + | Not applicable. | ||
<br> | <br> | ||
| − | |||
| − | |||
=== Glycan array === | === Glycan array === | ||
| − | F17G adhesins have been screened for their glycan specificity: http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query= | + | F17G adhesins have been screened for their glycan specificity (click [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_PA_v2_177_11182005 here]). To see all glycan array results for F17G adhesin, click [http://www.functionalglycomics.org/glycomics/search/jsp/result.jsp?query=F17G&cat=coreh here]. |
| + | |||
| + | == Related GBPs == | ||
| + | FedF [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=FedF&maxresults=20 (CFG data)], CfaE [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=CfaE&maxresults=20 (CFG data)], FimH [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=FimH&maxresults=20 (CFG data)], PapG, CupB6 [http://www.functionalglycomics.org/glycomics/search/jsp/landing.jsp?query=CupB6&maxresults=20 (CFG data)]. | ||
The specificity of some of the other fimbrial tip adhesins was determined by CFG glycan array analysis ([http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_2358 ''P. gingivalis'' fimbriae], [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_PA_v2_178_11182005 ''E. coli'' FedF adhesin], [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_1106 ''E. coli'' CfaE adhesin from CFA/I pili]) | The specificity of some of the other fimbrial tip adhesins was determined by CFG glycan array analysis ([http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_2358 ''P. gingivalis'' fimbriae], [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_PA_v2_178_11182005 ''E. coli'' FedF adhesin], [http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_1106 ''E. coli'' CfaE adhesin from CFA/I pili]) | ||
| − | |||
| − | |||
| − | |||
== References == | == References == | ||
Latest revision as of 11:42, 29 October 2011
The F17-G (GafD) adhesin at the tip of flexible F17 fimbriae of enterotoxigenic Escherichia coli mediates binding to N-acetyl-β-D-glucosamine-presenting receptors on the microvilli of the intestinal epithelium of ruminants, leading to diarrhea or septicaemia. F17-G belong to two-domain adhesins (TDA)s consisting of a pilin domain and a lectin domain, both having an Ig-fold joined via a short interdomain linker [1][2]. Related adhesins have been characterized in enteropathogenic E. coli ( FedF on F18 fimbriae[3] and CfaE on CFA/I pili[4]) ) and uropathogenic ones (FimH on type 1 fimbriae[5] and PapG on P-pili[6]). Fimbrial adhesins from other organisms, such as CupB6 from Pseudomonas aeruginosa are also investigated. All share the immunoglobulin-like fold of the two structural components, despite lack of any sequence identity and diversity in carbohydrate specificity and binding site, and the corresponding pili are assembled by the chaperone-usher pathway[7][8]. The paradigm is unique among TAD for his specificity toward GlcNAc. The binding site is located laterally and not at the tip of the pili, therefore the long and flexible F17 fimbriae could intrude between the microvilli of the epithelium, with the binding site of the lectin domain interacting laterally with GlcNAc-containing receptors. Five naturally occurring variants, differing in 1-18 amino acids of the adhesion domain have been identified[9].
CFG Participating Investigators contributing to the understanding of this paradigm
This is an emerging field of investigation and contributions arose from a small number of CFG Participating Investigators (PIs). These include: Esther Bullit, Eric Cox, Anne Imberty, Remy Loris, James Nataro
Progress toward understanding this GBP paradigm
This section documents what is currently known about F17G/GafD, its carbohydrate ligand(s), and how they interact to mediate cell communication.
Carbohydrate ligands
The F17G adhesin is most specific for the disaccharide GlcNAcb1,3Gal that can be recognised as a terminal or internal sequence in bovine glycophorin [10]:
![]()
The branched form gives a closely similar fluorescent signal:
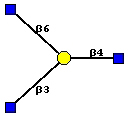
The presence of the b1,3 linkage of N-acetyl glucosamine to galactose enhances the affinity for F17G at least 2-fold, compared to the monosaccharide N-acetyl glucosamine, as validated using surface plasmon resonance measurements. Second best binders are the b1,4 and b1,6 galactose linked disaccharides, whereas chitobiose, that is also a characterized inhibitor of F17G-mediated bacterial adhesion, is clearly lagging behind. F17G can thus be ranked under glycan binding proteins that display high selectivity.
Cellular expression of GBP and ligands
F17G adhesins are expressed on enterotoxigenic E. coli infecting neonatal lambs, calves, and goat kids.
The F17G ligand GlcNAcb1,3Gal occurs universally but mostly internally in the sequence of poly-lactosaminyl glycans and blood group antigens.
These glycan structures are widely expressed on mamalian cell surfaces.
F17-fimbriated E. coli predominantly colonize neonatal animals, but also are a major causal agent (55%) of mastitis in bovines [11]. Congruent with the glycans recognized by F17G on the printed array versions 2.1 and 4.1, the N-acetyl glucosamine residue of GlcNAcb1,3Gal may be unsubstituted at the early life stage of calves, that are at the same time protected from bacterial infections by glycans secreted in the cow's milk.
Biosynthesis of ligands
The target sequence GlcNAcβ1-3Gal appears in O-linked glycans, where it is synthesized by UDP-GlcNAc:βGalβ1-3 GlcNAc transferase 3. Other enzymes that can synthesize this linkage on N- and O-linked glycans include
UDP-GlcNAc:βGal β1-3 GlcNAc transferase 4,
UDP-GlcNAc:βGal β1-3 GlcNAc transferase 5,
UDP-GlcNAc:βGal β1-3 GlcNAc transferase 6, and
UDP-GlcNAc:βGal β1-3 GlcNAc transferase 7.
Structure
The F17G adhesin is a two-domain adhesin (TDA) located at the F17 fimbrial tip. The determination of the crystal structure of the F17G lectin domain led to the discovery of the variable immunoglobulin-like structure as a paradigm for bacterial fimbrial TDAs [1]. F17G has a shallow groove for carbohydrate recognition on its flank.
Biological roles of GBP-ligand interaction
The F17G fimbrial lectin enhances intestinal colonization in the early life of ruminants. The long and flexible F17 fimbriae can penetrate deep between intestinal microvilli, where the fimbrial tip adhesin finds its glycan receptors. The subsequent secretion of heat stable and heat labile toxins can lead to severe diarrhea.
CFG resources used in investigations
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the CFG database search results for fimbriae and pili.
Glycan profiling
Glycogene microarray
F17G/GafD is not represented on the CFG microarrays, which only contain probes for mouse and human glycogenes.
Knockout mouse lines
Not applicable.
Glycan array
F17G adhesins have been screened for their glycan specificity (click here). To see all glycan array results for F17G adhesin, click here.
Related GBPs
FedF (CFG data), CfaE (CFG data), FimH (CFG data), PapG, CupB6 (CFG data).
The specificity of some of the other fimbrial tip adhesins was determined by CFG glycan array analysis (P. gingivalis fimbriae, E. coli FedF adhesin, E. coli CfaE adhesin from CFA/I pili)
References
- ↑ 1.0 1.1 Buts, L., Bouckaert, J., De Gents, E., Loris, R., Oscarson, S., Lahmann, M., Messens, J., Brosens, E., Wyns, L. & De Greve, H. (2003). The fimbrial adhesin F17-G of enterotoxigenic Escherichia coli has an immunoglobulin-like lectin domain that binds N-acetylglucosamine. Mol. Microb. 49, 705-715.
- ↑ Merckel, M. C., Tanskanen, J., Edelman, S., Westerlund-Wilkström, B., Korhonen, T. K. & Goldman, A. (2003). The structural basis of receptor-binding by Escherichia coli associaed with diarrhea and septicemia. J. Mol. Biol. 331, 897-905.
- ↑ Coddens, A., Diswall, M., Angstrom, J., Breimer, M. E., Goddeeris, B., Cox, E. & Teneberg, S. (2009). Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J Biol Chem 284, 9713-26.
- ↑ Poole, S. T., McVeigh, A. L., Anantha, R. P., Lee, L. H., Akay, Y. M., Pontzer, E. A., Scott, D. A., Bullitt, E. & Savarino, S. J. (2007). Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63, 1372-84.
- ↑ Bouckaert, J., Berglund, J., Schembri, M., De Gents, E., Cools, L., Wuhrer, M., Hung, C.-S., Pinkner, J., Slättegard, R., Savialov, A., Choudhury, D., Langermann, S., Hultgren, S. J., Wyns, L., Klemm, P., Oscarson, S., Knight, S. D. & De Greve, H. (2005). Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microb. 55, 441-455.
- ↑ Dodson, K. W., Pinkner, J. S., Rose, T., Magnusson, G., Hultgren, S. J. & Waksman, G. (2001). Structural basis of the interaction of the pyelonephritic E. coli adhesin to ist human kideny receptor. Cell 105, 733-743.
- ↑ De Greve, H., Wyns, L. & Bouckaert, J. (2007). Combining sites of bacterial fimbriae. Curr Opin Struct Biol 17, 506-12.
- ↑ Sauer, F. G., Barnhart, M., Choudhury, D., Knight, S. D., Waksman, G. & Hultgren, S. J. (2000). Chaperone-assisted pilus assembly and bacterial attachment. Curr Opin Struct Biol 10, 548-56.
- ↑ De Kerpel, M., Van Molle, I., Brys, L., Wyns, L., De Greve, H. & Bouckaert, J. (2006). N-terminal truncation enables crystallization of the receptor-binding domain of the FedF bacterial adhesin. Acta Crystallogr Sect F Struct Biol Cryst Commun 62, 1278-82.
- ↑ Mouricout, M., Milhavet, M., Durié, C., Grange, P. Characterization of glycoprotein glycan receptors for Escherichia coli F17 fimbrial lectin, Microb. Pathog. (1995) 18, 297-306
- ↑ Lipman, L.J.A., de Nijs A., Gaastra W.. Isolation and identification of fimbriae and toxin production by Escherichia coli strains from cows with clinical mastitis, Vet. Microbiology 47 (1995) p. 1-7
Acknowledgements
The CFG is grateful to the following PIs for their contributions to this wiki page: Alisdair Boraston, Julie Bouckaert, Anne Imberty