Difference between revisions of "Influenza hemagglutinin H3"
Gillian Air (talk | contribs) |
Gillian Air (talk | contribs) |
||
| Line 43: | Line 43: | ||
<br> | <br> | ||
=== Knockout mouse lines === | === Knockout mouse lines === | ||
| + | Unfortunately the mouse is a very poor model of influenza infection. Some viruses with H3 HA infect mice quite readily, but do not cause a human-like disease. This means that studies of infection and transmission of H3N2 influenza viruses in SiaT knockout mice are difficult to translate to the human disease. | ||
| + | <br> | ||
| − | |||
=== Glycan array === | === Glycan array === | ||
The majority of PI-initiated requests for CFG resources to study influenza have been requests for analysis of receptor specificity on the glycan array, and the remainder have been requests for compounds to conduct ''in vitro'' assays in investigators laboratories. In addition, the CFG glycan array library has been used for custom sialic acid glycan array to the U.S. Centers for Disease Control (CDC) for analysis of the receptor specificity of emerging viruses, with data deposited to the CFG database. | The majority of PI-initiated requests for CFG resources to study influenza have been requests for analysis of receptor specificity on the glycan array, and the remainder have been requests for compounds to conduct ''in vitro'' assays in investigators laboratories. In addition, the CFG glycan array library has been used for custom sialic acid glycan array to the U.S. Centers for Disease Control (CDC) for analysis of the receptor specificity of emerging viruses, with data deposited to the CFG database. | ||
Revision as of 05:37, 13 June 2010
Influenza hemagglutinin (H3 serotype) was the first glycoprotein structure to be solved at atomic resolution, by Ian Wilson, John Skehel and Don Wiley in 1981. The collaboration between the Skehel and Wiley labs provided great insight into hemagglutinin function, and it remains the prototype for understanding receptor recognition, antigenic variation, and the extraordinary conformational changes associated with target membrane insertion and ultimately fusion of viral with cell membrane to allow the viral genome to enter the cell and replicate.
In the 1980s the Paulson lab made the seminal discovery that human and avian viruses with the H3 serotype have different receptor specificities; that human viruses bind to Neu5Acα2-6Gal while avian viruses bind Neu5Acα2-3Gal. In two very elegant experiments they were able to switch these specificities by applying selective pressure, and showed that a single amino acid change (L226Q) was all that was required for early H3N2 viruses to switch between human and avian specificities. These results showed how easy it can be for avian viruses to cross the species barrier into humans. Seasonal influenza viruses with the H3 serotype continue to circulate in the human population, and subtleties in their receptor specificities appear to be playing a role in how clinical isolates can be recovered in laboratory hosts. CFG investigators are using tools provided by the CFG to analyze the detailed receptor specificity of the circulating H3N2 influenza viruses and their interaction with laboratory hosts to better understand this phenomenon, which has direct consequences on production of vaccines.
Although the influenza H3 hemagglutinin has been chosen as the paradigm, since so much is known, there are 16 subtypes of influenza HA (H1-H16), defined by lack of antigenic cross-reactivity. There is typically only about 20% amino acid sequence identity between HAs of different subtypes. There are interesting and important differences in how easily a particular strain within the subtype can change its binding specificity between avian-like and human-like receptors, leading to the failure of H5N1 to be established in the human population while swine-origin H1N1 showed high transmissibility between humans from the time it was first isolated.
To understand the transmission of influenza viruses and how new pandemics begin, it will be important to study a variety of HA subtypes and strains. but for other subtypes the rules are different and are not yet understood. The H5N1 avian virus has still not acquired the ability to transmit between humans, despite at least 15 years of opportunity. The CFG has facilitated considerable advances in our knowledge of the role of sialic acid binding in influenza host specificity and tropism for the upper or lower respiratory tract, and these studies need to be continued until we understand how influenza viruses enter the human population to cause each new pandemic, and the role of receptor specificity in pathogenicity.
CFG Participating Investigators contributing to the understanding of this paradigm
CFG Participating Investigators (PIs) contributing to the understanding of H3 include: Gillian Air, Rafi Ahmed, Nicolai Bovin, Ruben Donis, Chwan-Chuen King, Vladimir Lugovtsev, Christopher Olsen, Peter Palese, James Paulson, Andrew Pekosz, Daniel Perez, Peter P.J.M. Rottier, Charles Russell, Ram Sasisekharan, Dorothy Scott, David Smith, James Stevens, Stephen Mark Tompkins, Reinhard Vlasak, Qinghua Wang, Ian Wilson
Progress toward understanding this GBP paradigm
Carbohydrate ligands
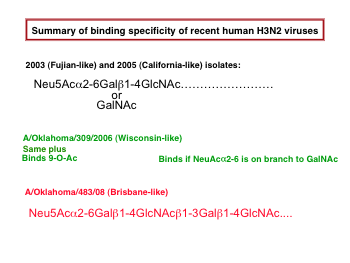
Ligands for H3 hemagglutinin are sialylated glycans. The H3 hemagglutinin of human viruses (subtype H3N2) binds to N-acetylneuraminic acid linked α2-6 to galactose, sometimes N-acetylgalactosamine. Recent human H3 HAs have shown variation in their specificity of binding downstream sugars[1]
Cellular expression
HA is expressed on the surface of influenza virus infected cells before being budded out into progeny virions. H3N2 viruses infect the respiratory tract of humans and birds; in birds they may also infect the gut epithelia. H3N2 viruses infect very few continuous cell lines. Madin-Darby canine kidney cells are most commonly used. Non-permissive cell lines may take up virus efficiently, replicate RNA and express HA on the cell surface but do not bud new virus particles [2]
Structure
The crystal structure of H3 HA was determined by Wilson, Wiley & Skehel in 1981. This has served as a model for more recent HA structure determinations such as H1 HA [3]
Biological roles of GBP-ligand interaction
Sialylated glycans on the surface of cells lining the respiratory tract serve to capture virus to initiate infection. Glycan array analyses have confirmed that human influenza viruses such as those carrying the H3 HA bind only to structures with NeuAcα2-6 and avian isolates bind only to structures containing NeuAcα2-3. The role of this GBP-glycan interaction in initiating endocytosis and replication is still unclear.
CFG resources used in investigations
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the CFG database search results for "hemagglutinin".
Glycan profiling
Virologists have used lectin binding to try to determine where the influenza virus receptors specific for human or avian HAs are located in the human respiratory tract, with mixed results [4]. A complete profile of human trachea as well as lung is needed.
Glycogene microarray
Knockout mouse lines
Unfortunately the mouse is a very poor model of influenza infection. Some viruses with H3 HA infect mice quite readily, but do not cause a human-like disease. This means that studies of infection and transmission of H3N2 influenza viruses in SiaT knockout mice are difficult to translate to the human disease.
Glycan array
The majority of PI-initiated requests for CFG resources to study influenza have been requests for analysis of receptor specificity on the glycan array, and the remainder have been requests for compounds to conduct in vitro assays in investigators laboratories. In addition, the CFG glycan array library has been used for custom sialic acid glycan array to the U.S. Centers for Disease Control (CDC) for analysis of the receptor specificity of emerging viruses, with data deposited to the CFG database.
Related GBPs
Influenza virus HAs of other serotype H1, H2, H4, H5, H6, H7, H8, H9, H10, H11, H12, H13, H14, H15, H16 and type B. Type A subtypes H1, H2, H5, H6, H7, and H9 are all being actively investigated by CFG investigators for their potential to jump to humans and type B for its failure to spread in non-human species.
References
- ↑ Gulati S, Smith DF, Air GM. Deletions of neuraminidase and resistance to oseltamivir may be a consequence of restricted receptor specificity in recent H3N2 influenza viruses. Virology J 2009;6(22).
- ↑ Kumari K, Gulati S, Smith DF, Gulati U, Cummings RD, Air GM. Receptor binding specificity of recent human H3N2 influenza viruses. Virol J 2007;4(42):1-12.
- ↑ Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, Wilson IA. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010 Apr 16;328(5976):357-60.
- ↑ Nicholls JM, Chan RW, Russell RJ, Air GM, Peiris JS. Evolving complexities of influenza virus and its receptors. Trends Microbiol 2008 2008 Apr;16(4):149-57.
Acknowledgements
The CFG is grateful to the following PIs for their contributions to this wiki page: Gillian Air, James Paulson