Difference between revisions of "CD22"
m |
Jim Paulson (talk | contribs) |
||
| Line 1: | Line 1: | ||
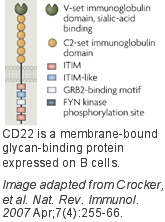
| − | CD22 is predominantly expressed on B cells and is well documented as a regulator of B cell receptor (BCR) signaling<ref name="Crocker 2007">Crocker PR, Paulson JC, Varki A. [http://www.ncbi.nlm.nih.gov/pubmed/17380156 Siglecs and their roles in the immune system]. ''Nat Rev Immunol'' 2007 Apr;7(4):255-66. Review.</ref>. It is one of four siglecs that are highly conserved among mammals. This paradigm is unique among the siglecs in that the cytoplasmic domain has six conserved tyrosine motifs, including three immunoreceptor tyrosine inhibitory motifs (ITIM), one ITIM-like motif, and a growth factor receptor bound protein2 (GRB2) motif. These tyrosine motifs are involved in regulation of BCR signaling and also mediate its constitutive clathrin mediated endocytosis, an activity believed to be tied to its regulation of cell signaling. The preferred glycan ligand of CD22 differs significantly in humans and mice<ref name="Crocker 2007"/><ref>Kimura N, Ohmori K, Miyazaki K, Izawa M, Matsuzaki Y, Yasuda Y, Takematsu H, Kozutsumi Y, Moriyama A, Kannagi R. [http://www.ncbi.nlm.nih.gov/pubmed/17728258 Human B-lymphocytes express alpha2-6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2]. J'' Biol Chem''. 2007 Nov 2;282(44):32200-7.</ref><ref>Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. [http://www.ncbi.nlm.nih.gov/pubmed/15563589 Printed covalent glycan array for ligand profiling of diverse glycan binding proteins]. ''Proc Natl Acad Sci U S A''. 2004 Dec 7;101(49):17033-8.</ref>. While both recognize the sequence Siaa-2-6Galb-1-4GlcNAc expressed abundantly on B cells, murine CD22 prefers Neu5Gc (not found in humans) over Neu5Ac, while human CD22 exhibits highest affinity for sulfated sialoside, Neu5Aca-2-6Galb-1-4[6S]GlcNAc, demonstrating significant evolution of ligand specificity with conservation of function. Although CD22 recognizes ligands on the same cell in ''cis'', it also binds to ligands in ''trans'' if expressed on adjacent contacting cells. A major area of investigation is to understand the relative roles of ''cis'' and ''trans'' ligands in CD22 function. | + | CD22 is predominantly expressed on B cells and is well documented as a regulator of B cell receptor (BCR) signaling<ref name="Crocker 2007">Crocker PR, Paulson JC, Varki A. [http://www.ncbi.nlm.nih.gov/pubmed/17380156 Siglecs and their roles in the immune system]. ''Nat Rev Immunol'' 2007 Apr;7(4):255-66. Review.</ref>. It is one of four siglecs that are highly conserved among mammals. This paradigm is unique among the siglecs in that the cytoplasmic domain has six conserved tyrosine motifs, including three immunoreceptor tyrosine inhibitory motifs (ITIM), one ITIM-like motif, and a growth factor receptor bound protein2 (GRB2) motif. These tyrosine motifs are involved in regulation of BCR signaling and also mediate its constitutive clathrin mediated endocytosis, an activity believed to be tied to its regulation of cell signaling. The preferred glycan ligand of CD22 differs significantly in humans and mice<ref name="Crocker 2007"/><ref name="Kimura 2007">Kimura N, Ohmori K, Miyazaki K, Izawa M, Matsuzaki Y, Yasuda Y, Takematsu H, Kozutsumi Y, Moriyama A, Kannagi R. [http://www.ncbi.nlm.nih.gov/pubmed/17728258 Human B-lymphocytes express alpha2-6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2]. J'' Biol Chem''. 2007 Nov 2;282(44):32200-7.</ref><ref>Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. [http://www.ncbi.nlm.nih.gov/pubmed/15563589 Printed covalent glycan array for ligand profiling of diverse glycan binding proteins]. ''Proc Natl Acad Sci U S A''. 2004 Dec 7;101(49):17033-8.</ref>. While both recognize the sequence Siaa-2-6Galb-1-4GlcNAc expressed abundantly on B cells, murine CD22 prefers Neu5Gc (not found in humans) over Neu5Ac, while human CD22 exhibits highest affinity for sulfated sialoside, Neu5Aca-2-6Galb-1-4[6S]GlcNAc, demonstrating significant evolution of ligand specificity with conservation of function. Although CD22 recognizes ligands on the same cell in ''cis'', it also binds to ligands in ''trans'' if expressed on adjacent contacting cells. A major area of investigation is to understand the relative roles of ''cis'' and ''trans'' ligands in CD22 function. |
[[Image:SiglecCD22.jpg|right|alt text]] | [[Image:SiglecCD22.jpg|right|alt text]] | ||
| Line 9: | Line 9: | ||
=== Carbohydrate ligands === | === Carbohydrate ligands === | ||
| − | + | Although CD22 is highly conserved throughout mammalian species, murine and human CD22 are known to exhibit significant differences in their specificities that appear to have evolved to compensate for changes in the glycan ligands expressed on B cells. While both bind Siaα2-6Gal terminated glycans, murine CD22 prefers NeuGc (NeuGcα2-6Galβ1-4GlcNAc), which is not found in humans. In contrast, human human CD22 recognizes NeuAc and NeuGc with equal affinity. In addition, however, human CD22 exhibits highest affinity for a ligand with sulfate at the 6 position of GlcNAc (NeuAcα2-6Galβ1-4[6S]GlcNAc).<ref name="Crocker 2007"/><ref name="Kimura 2007"/> 9-O-acetylation of sialic acid abrogates binding of CD22, which is thought to regulate the binding of ''cis'' ligands on B cells. | |
<br> | <br> | ||
=== Cellular expression of GBP and ligands === | === Cellular expression of GBP and ligands === | ||
| − | + | CD22 is primarily expressed on mature B cells and to a lesser extent on memory B cells. However, it is not expressed on pre-B cells and differentiated plasma cells. Like many siglecs, CD22 interacts with endogenous ligands on B cells in ''cis'', and on other cells, such as T cells and bone marrow vessel endothelial cells in ''trans''. Although ''cis'' ligands of tend to mask the CD22 binding site, CD22 is able to interact with ''trans'' ligands on contacting cells (B cells and T cells), and to bind to synthetic multivalent ligands that have sufficient avidity. | |
<br> | <br> | ||
=== Biosynthesis of ligands === | === Biosynthesis of ligands === | ||
| − | + | The ligands of CD22 are predominately the product of a single sialyltransferase, ST6Gal I. Mice deficient in ST6Gal I express no ligands on B cells resulting in an immuno-deficient phenotype. | |
<br> | <br> | ||
=== Structure === | === Structure === | ||
| − | + | Although the crystal structure of CD22 has not yet been elucidated, structures of other siglecs, including sialoadhesin, siglec-5 and siglec-7 have shed insights into the nature of the ligand binding site of CD22.<ref name="Crocker 2007"/> | |
<br> | <br> | ||
=== Biological roles of GBP-ligand interaction === | === Biological roles of GBP-ligand interaction === | ||
| Line 27: | Line 27: | ||
=== Glycan profiling === | === Glycan profiling === | ||
| − | |||
<br> | <br> | ||
=== Glycogene microarray === | === Glycogene microarray === | ||
| − | The CFG glycogene microarray has been used to show that | + | The CFG glycogene microarray has been used to show that ST6Gal I is downregulated [https://www.functionalglycomics.org/glycomics/publicdata/microarray.jsp?resReqId=cfg_rRequest_2 'on T cells] upon activation suggesting that B cell ''trans'' ligands are reduced on activated T cells. |
=== Knockout mouse lines === | === Knockout mouse lines === | ||
| − | Mice deficient in [https://www.functionalglycomics.org/static/consortium/resources/resourcecoref16.shtml CD22] and the sialyltransferase responsible for synthesis of its ligands ([https://www.functionalglycomics.org/glycomics/publicdata/phenotyping.jsp ST6Gal I]) distributed by the CFG have been instrumental in understanding the biology of CD22. | + | Mice deficient in [https://www.functionalglycomics.org/static/consortium/resources/resourcecoref16.shtml CD22] and the sialyltransferase, ST6Gal I, responsible for synthesis of its ligands ([https://www.functionalglycomics.org/glycomics/publicdata/phenotyping.jsp ST6Gal I]) distributed by the CFG have been instrumental in understanding the biology of CD22. |
=== Glycan array === | === Glycan array === | ||
Revision as of 01:25, 20 July 2010
CD22 is predominantly expressed on B cells and is well documented as a regulator of B cell receptor (BCR) signaling[1]. It is one of four siglecs that are highly conserved among mammals. This paradigm is unique among the siglecs in that the cytoplasmic domain has six conserved tyrosine motifs, including three immunoreceptor tyrosine inhibitory motifs (ITIM), one ITIM-like motif, and a growth factor receptor bound protein2 (GRB2) motif. These tyrosine motifs are involved in regulation of BCR signaling and also mediate its constitutive clathrin mediated endocytosis, an activity believed to be tied to its regulation of cell signaling. The preferred glycan ligand of CD22 differs significantly in humans and mice[1][2][3]. While both recognize the sequence Siaa-2-6Galb-1-4GlcNAc expressed abundantly on B cells, murine CD22 prefers Neu5Gc (not found in humans) over Neu5Ac, while human CD22 exhibits highest affinity for sulfated sialoside, Neu5Aca-2-6Galb-1-4[6S]GlcNAc, demonstrating significant evolution of ligand specificity with conservation of function. Although CD22 recognizes ligands on the same cell in cis, it also binds to ligands in trans if expressed on adjacent contacting cells. A major area of investigation is to understand the relative roles of cis and trans ligands in CD22 function.
CFG Participating Investigators contributing to the understanding of this paradigm
CFG Participating Investigators (PIs) have made major contributions to the understanding of the biology of human and murine CD22. These include: Nicolai Bovin, Paul Crocker, Jamey Marth, David Nemazee, Lars Nitschke, Jim Paulson, Ajit Varki
Progress toward understanding this GBP paradigm
Carbohydrate ligands
Although CD22 is highly conserved throughout mammalian species, murine and human CD22 are known to exhibit significant differences in their specificities that appear to have evolved to compensate for changes in the glycan ligands expressed on B cells. While both bind Siaα2-6Gal terminated glycans, murine CD22 prefers NeuGc (NeuGcα2-6Galβ1-4GlcNAc), which is not found in humans. In contrast, human human CD22 recognizes NeuAc and NeuGc with equal affinity. In addition, however, human CD22 exhibits highest affinity for a ligand with sulfate at the 6 position of GlcNAc (NeuAcα2-6Galβ1-4[6S]GlcNAc).[1][2] 9-O-acetylation of sialic acid abrogates binding of CD22, which is thought to regulate the binding of cis ligands on B cells.
Cellular expression of GBP and ligands
CD22 is primarily expressed on mature B cells and to a lesser extent on memory B cells. However, it is not expressed on pre-B cells and differentiated plasma cells. Like many siglecs, CD22 interacts with endogenous ligands on B cells in cis, and on other cells, such as T cells and bone marrow vessel endothelial cells in trans. Although cis ligands of tend to mask the CD22 binding site, CD22 is able to interact with trans ligands on contacting cells (B cells and T cells), and to bind to synthetic multivalent ligands that have sufficient avidity.
Biosynthesis of ligands
The ligands of CD22 are predominately the product of a single sialyltransferase, ST6Gal I. Mice deficient in ST6Gal I express no ligands on B cells resulting in an immuno-deficient phenotype.
Structure
Although the crystal structure of CD22 has not yet been elucidated, structures of other siglecs, including sialoadhesin, siglec-5 and siglec-7 have shed insights into the nature of the ligand binding site of CD22.[1]
Biological roles of GBP-ligand interaction
CFG resources used in investigations
The best examples of CFG contributions to this paradigm are described below, with links to specific data sets. For a complete list of CFG data and resources relating to this paradigm, see the CFG database search results for CD22.
Glycan profiling
Glycogene microarray
The CFG glycogene microarray has been used to show that ST6Gal I is downregulated 'on T cells upon activation suggesting that B cell trans ligands are reduced on activated T cells.
Knockout mouse lines
Mice deficient in CD22 and the sialyltransferase, ST6Gal I, responsible for synthesis of its ligands (ST6Gal I) distributed by the CFG have been instrumental in understanding the biology of CD22.
Glycan array
The CFG's glycan array was instrumental in identification of the high affinity ligands of CD22 as sialylated-sulfated glycans.
Related GBPs
None. This paradigm is unique among the siglecs in that the cytoplasmic domain has six conserved tyrosine motifs, including three immunoreceptor tyrosine inhibitory motifs (ITIM), one ITIM-like motif, and a growth factor receptor bound protein2 (GRB2) motif.
References
- ↑ 1.0 1.1 1.2 1.3 Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol 2007 Apr;7(4):255-66. Review.
- ↑ 2.0 2.1 Kimura N, Ohmori K, Miyazaki K, Izawa M, Matsuzaki Y, Yasuda Y, Takematsu H, Kozutsumi Y, Moriyama A, Kannagi R. Human B-lymphocytes express alpha2-6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2. J Biol Chem. 2007 Nov 2;282(44):32200-7.
- ↑ Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17033-8.
Acknowledgements
The CFG is grateful to the following PIs for their contributions to this wiki page: Paul Crocker, James Paulson